What to Do When Rad Seq Shows a Lot of Divergence
Abstract
Speciation encompasses a continuum over time from freely interbreeding populations to reproductively isolated species. Along this process, ecotypes – the event of local adaptation – may be on the route to new species. Nosotros investigated whether three autotetraploid Cochlearia officinalis ecotypes, adapted to dissimilar habitats (embankment, estuary, spring), are genetically differentiated and result from parallel ecotypic deviation in 2 singled-out geographical regions. We obtained genetic data from thousands of single nucleotide polymorphisms (SNPs) from restriction-site associated DNA sequencing (RADseq) and from six microsatellite markers for 12 populations to assess genetic divergence at ecotypic, geographic and population level. The genetic patterns support differentiation amidst ecotypes as suggested by morphology and environmental. The data fit a scenario where the ancestral beach ecotype has recurrently and polytopically given ascension to the estuary and spring ecotypes. Several ecologically-relevant loci with consequent non-random segregating patterns are identified across the recurrent origins, in particular around genes related to common salt stress. Despite existence ecologically singled-out, the Cochlearia ecotypes still represent an early phase in the procedure of speciation, as reproductive isolation has not (all the same) adult. A sequenced annotated genome is needed to specifically target candidate genes underlying local adaptation.
Introduction
Speciation oft occurs as a continuous process over time from freely interbreeding populations to reproductively isolated species1,two,3. Along this continuum, ecotypes may be formed as a result of local adaptation to specific sets of environmental factors that define different habitats4. Fifty-fifty though the ecotype concept and its office in plant speciation have been subject to heavy contend during the last century2, several empirical studies show non-random organisation of morphological and genetic variation related to more or less steep ecological gradients4,5,vi, supporting that ecotypes could be considered non-static entities along the speciation continuum. The study of adaptive difference between ecotypes may, thus, exist an important contribution for understanding the process of speciation. Instances of parallel ecotypic deviation where adaptation to similar atmospheric condition repeatedly cause similar phenotypic changes in closely related organisms are especially useful for disentangling the respective roles of drift and natural selection in shaping genomic divergence amid genomes and for studying the genes underlying local adaptation7. The ecological variation plant among populations of the autotetraploid Cochlearia officinalis in northern Norway potentially represents a highly valuable system to explore parallel ecotypic differentiation in plants.
The genus Cochlearia (Brassicaceae) constitutes a good instance of a group of recently evolved, and in some cases not yet fully differentiated taxa, which most probable diversified during the mid or tardily Pleistocene8,9,10. The taxa inhabit coastal and inland (alpine) habitats and are distributed throughout Central and Northern Europe, extending the distribution of the genus into the chill region11. Most taxa are dependent on a good supply of water or moist soil atmospheric condition throughout the twelvemonth, and parallel adaptation to different types of moist habitats may exist important for the diversification within the grouping12, thirteen. The taxa together exhibit complex variation not only with regard to environmental and morphology, just constitute also a polyploid complex of diploids, tetraploids, hexaploids, and octoploids9, 10, 14,15,sixteen,17,18.
The tetraploid C. officinalis is a common cold-tolerant halophyte, widely distributed along the European coastline14, 19, 20. Gill suggested based on studies of chromosome associations during meiosis in F1 hybrids, that C. officinalis originated by autopolyploidy from the Central European C. pyrenaica 17. Molecular data support an autotetraploid origin, although not directly from present day diploids10. Previous studies have found morphological and ecological variation in Northern Scandinavia, which has been suggested to represent differentiation at the ecotypic level, and several subspecies accept been recognised16, 20, 21. The common embankment ecotype, or ssp. officinalis, grows in gravel beaches (Fig. 1a), crevices in beach cliffs (Supporting Information Fig. S1a), salt marshes and occasionally in bird cliffs, where it shows vigorous growth and seems to be adapted to exploit the high nutrient levels13, 16. The estuary ecotype, or ssp. norvegica, grows in sheltered habitats near outlets of large rivers in innermost fjords (Fig. 1b, Supporting Information Fig. S1b), which are inundated by brackish water at flood-tide13, sixteen. This ecotype seems to be adapted to handle nutrient poor habitats and shows very piddling increase in growth when presented with higher nitrogen levels21. The bound ecotype, or ssp. integrifolia, grows inland in more than or less base of operations-rich common cold springs (Fig. 1c, Supporting Information Fig. S1c), along streams and brooks or in snow beds13, 16.

Habit and habitat of the three ecotypes of Cochlearia officinalis (2n =24) in Troms, Kingdom of norway. (a) The embankment ecotype (ssp. officinalis) at localities Sjøvassbotn (left) and Skittenelv (right), (b) the estuary ecotype (ssp. norvegica) at locality Skibotn, and (c) the spring ecotype (ssp. integrifolia) at locality Kvaløysletta. (Photo: G.One thousand. Brandrud).
Nordal and Stabbetorp institute that the iii ecotypes are not only ecologically differentiated simply also to some caste morphologically distinct16. However, no single quantitative character unambiguously separates the 3 ecotypes16. The morphologically most distinct ecotype is the estuary ecotype, with larger flowers and cuneate (as opposed to more than or less kidney-shaped) rosette leaves that are fleshier than those of the other 2 ecotypes. In the beach ecotype, the fruit (silicula) is more spherical in outline than in the estuary and spring ecotypes. The spring ecotype has a tendency to be perennial rather than biennial as is the case for the two other ecotypes. This is indicated by a branching rhizome that gives rising to more rosettes, and by the evolution of buds earlier the snowfall has melted. When comparing plants collected in the field with plants cultivated in common atmospheric condition, distinctiveness in flower and fruit characters tended to be stable, whereas the size and shape of rosette and stalk leaves, also equally the branching and elongation of inflorescences, were highly plastic depending on environmental conditionsxvi.
Genetic studies of Cochlearia so far10, fourteen, 22,23,24,25 take not included plants representing the ecotypic variation found in Northern Scandinavia. Using several thou single nucleotide polymorphisms (SNPs) obtained from restriction-site associated DNA sequencing (RADseq), and microsatellite markers, we explore here whether and to what degree the ecotypes are genetically differentiated. We infer the genetic structure of the three ecotypes from 2 geographical areas of Northern Kingdom of norway and we enquire whether the ecological and morphological variation that we see today is the issue of parallel evolution through local adaptation to different habitats.
Results
RADseq analyses
Plant textile of the three C. officinalis ecotypes (beach, estuary and spring) were sampled in Northern Norway in ii areas (Fig. 2, Table one) where they broadly co-occur: Tromsø-Skibotn in Troms county (in the following called Troms) and Lofoten in Nordland county (in the following chosen Lofoten). Fifty-four individuals from 12 populations, representing the three ecotypes (Table 1), were analysed by menstruation cytometry to estimate ploidal level, and all were confirmed to be tetraploid.
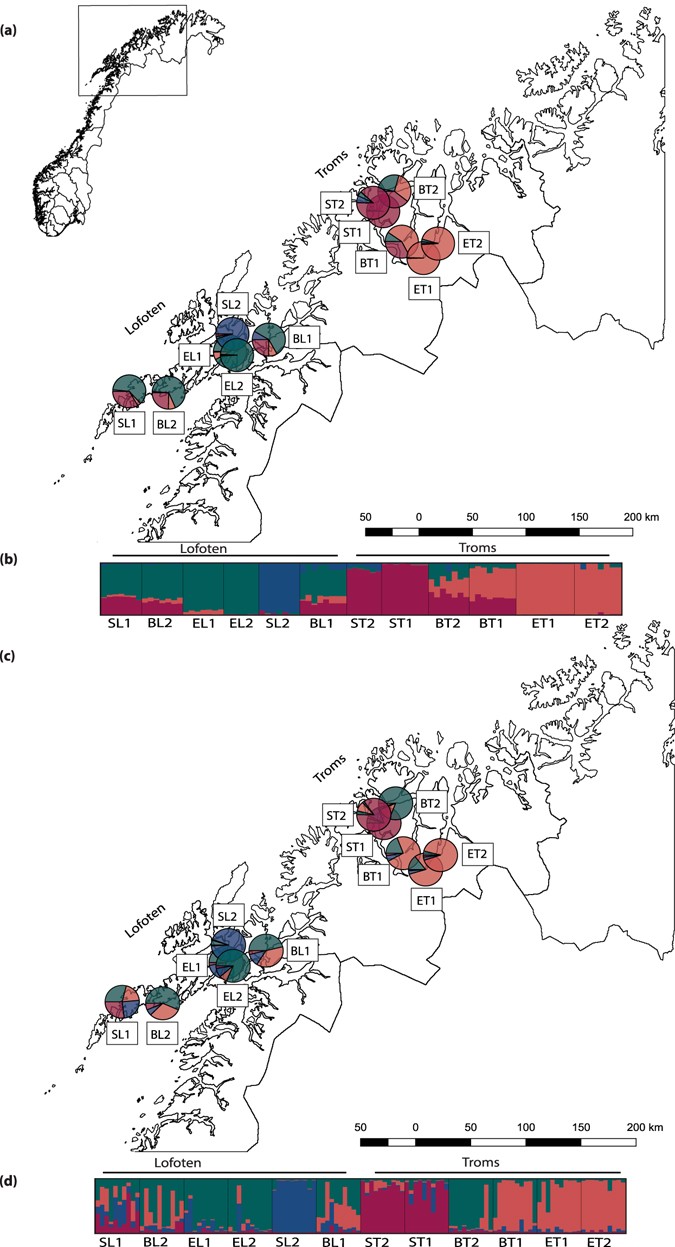
Genetic construction of 12 populations of Cochlearia officinalis in Northern Norway. (a,b) Results from Structure analysis (K = 4) performed on 89 individuals, using 4,296 SNPs from the RADseq information. (c,d) Results from Construction analysis (K = 4) performed on 120 individuals, using microsatellite allele sizes. (a,c) Overall assignment of populations to the four Structure groups visualized past pie charts on a map of Northern Norway. The 2 sampling areas are indicated as Troms and Lofoten. (b,d) Proportional assignment of individuals to the four STRUCTURE groups. Each individual is represented past a bar and populations are separated past a black line. Populations are named according to ecotype (B = embankment, E = estuary, S = spring) and geography (T = Troms, Fifty = Lofoten), meet Tabular array 1. The map layer was extracted from GADM version ane.072.
From c. 428 one thousand thousand raw paired-cease reads obtained from RADseq, c. 163 million forrad reads were retained after demultiplexing and cleaning. The 2d reads in the pairs were only used in the process of demultiplexing based on combinatorial inline barcodes and for extending contigs for the annotation of outlier loci. After de novo catalog edifice and SNP calling, we retained c. 15.000 loftier-quality loci present in at least 80% of the 89 individuals included in the analysis (Table 1). These were further filtered with various parameters to construct input files for population genetic and phylogenetic analyses (Supporting Information Table S1). Given that the 1C genome size of C. officinalis is estimated to 0.75 pg26, i.e. 734 megabases (Mbp), and following the procedure in Lowry et al.27, the retained RAD loci density in the electric current study was estimated to be 5,329 RAD loci over 734 Mbp, i.east. 7.26 RAD loci/Mbp.
The number of private alleles were highest in the beach ecotype (661) compared to the estuary (603) and bound (494) ecotypes (Supporting Information Table S2). All populations had negative inbreeding coefficients (FIS) when calculated from the RADseq information, indicating an excess of heterozygotes, estimates confirmed besides with our microsatellite analyses (run into beneath). It should be noted that when analysing RADseq data in STACKS, FIS is calculated as 1-(Ho/Heast). For polyploids we would, however, expect college levels of heterozygosity than for diploids28, significant that in our calculations FIS is most likely underestimated by using this approach. The spring population from Sørfjorddalen in Lofoten (SL2) had a lower number of private alleles, and an inbreeding coefficient closer to zero than other populations. This population grows in a bound in an open up forest area relatively far from the bounding main (Fig. S1c). The second spring population from Lofoten (Himmeltind, SL1), which grows in a small stream near the outlet to the sea, had a college number of private alleles and a more negative inbreeding coefficient, comparable to what we found for the embankment ecotype in the same surface area (Supporting Information Table S2).
The number of migrants based on individual alleles (Nm) was subunitary in all population pairs, suggesting an important role for drift (and/or local choice) in shaping the genetic structure of the group. In full general, the beach populations had highest connectivity, independent of geographic distance. The other ecotypes were less connected past cistron flow (except for the estuary populations from Troms), with the number of migrants inside ecotypes not different from that between ecotypes or betwixt regions. The lowest levels of gene period were found for the isolated jump population from Lofoten (SL2), followed by the two spring populations from Troms. Highest number of migrants was constitute between the two estuary populations from Troms, which are both geographically and genetically close, followed by the embankment populations from both areas (Supporting Data Fig. S2, Table S3).
Analyses of molecular variance (AMOVA; Supporting Data Table S4) showed that virtually of the variation in the dataset was constitute inside populations (with heterozygosity equally an of import office of the total variation, but come across higher up comments on FIS estimates). Although simply a modest percent (c. 5%) was explained by differences between ecotypes, this role was larger than the variation explained by the ii geographic regions (2%).
In a principal component analysis (PCA), based on four,296 SNPs, the first three axes explained 11.3% of the variation in the data (Fig. 3). Taken together, the three axes separated all populations except for some overlap between the two estuary populations from Troms and between the two beach populations from Troms. Further, the main signal followed the ecotypic differentiation (the outset and tertiary axes in combination) in addition to a (weaker) geographical separation between Troms and Lofoten (starting time and second axis in combination). Overall, the embankment populations were less well separated and localised at the centre of the plot. The isolated spring population from Lofoten (SL2) was the most distinct population, whereas the more exposed spring population from Lofoten (SL1) overlapped with one of the embankment populations from the aforementioned surface area. Although more vaguely, the same tendency was seen in Troms; the isolated forest spring population (Tromsdalen, ST1) was genetically more distinct from the beach populations than the spring population growing in a somewhat more exposed area (Kvaløysletta, ST2; Fig. 1c).
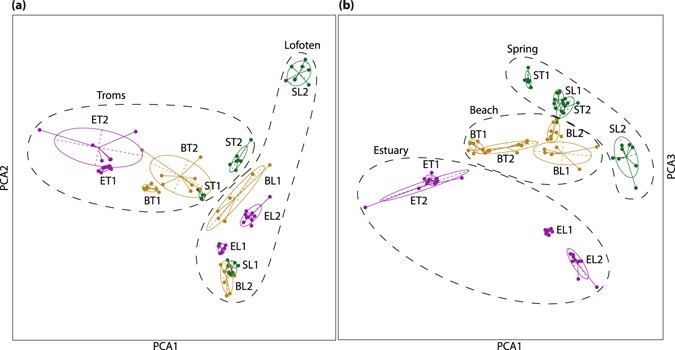
PCA performed on 12 populations of Cochlearia officinalis, using 4,296 SNPs from the RADseq data. (a) The beginning and second PCA axes. (b) The commencement and third PCA axes. The first PCA axis explains four.05%, the second axis 3.82%, and the third centrality iii.46% of the full variation. Color represents ecotypes: estuary - purple, beach - yellow and spring - green. Individuals (dots) are linked to the centre of the 95% inertia ellipse for the population to which they belong. Populations are named according to ecotype (B = beach, Due east = estuary, S = leap) and geography (T = Troms, 50 = Lofoten), see Table 1.
From a Construction analysis, based on 4,296 SNPs, K = iv was selected based on the optimal deltaK and the mean likelihood value (followed by Thousand = 2 and K = 9, Supporting Data Fig. S3). When the STRUCTURE results were plotted on a map of Northern Norway every bit pie charts representing the amalgamation of single populations to the four Structure groups (Fig. 2a), a geographical design was seen with ii of the groups ('majestic' and 'orange') dominating in Troms, and the other ii groups ('blue' and 'greenish') dominating in Lofoten. Overall, the beach populations were genetically similar and showed admixture of 3 of the 4 Construction groups, though with populations in Lofoten and Troms differing in relative resource allotment to each gene pool (Fig. 2b). The estuary populations in Lofoten allocated to a Construction group dominating among the embankment populations in Lofoten ('light-green'), whereas the estuary populations in Troms allocated to a STRUCTURE grouping dominating among the beach populations in Troms ('majestic'). The isolated spring population from Lofoten (SL2) was likewise in this analysis genetically the almost singled-out of all analysed populations, constituting a genetic group of its ain ('blue'), whereas the 2nd bound population from Lofoten (SL1) was admixed and genetically like to the embankment populations from the same area.
In a neighbor net, based on 4,311 SNPs, the individuals clustered co-ordinate to the 12 populations (Fig. four). The spring and estuary populations were mainly supported past well-defined splits, whereas the embankment populations were less well defined with relatively short splits and a loftier degree of reticulation corresponding to the high level of admixture seen in the Structure results. This was too the case for the admixed spring population from Lofoten (SL1). The isolated bound population from Lofoten (SL2) was again the most singled-out population, supported past the largest split in the network. There was a relatively clear geographical separate across the network, separating populations from the 2 sampling areas (Troms and Lofoten).
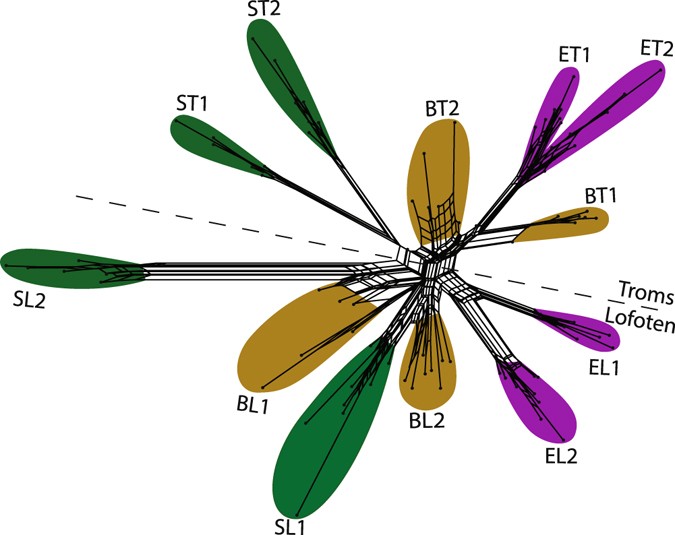
Neighbour net performed on 12 populations of Cochlearia officinalis, using four,311 SNPs from the RADseq information. Colour represents ecotypes: beach - yellow, estuary - majestic and spring - dark-green. Populations are named according to ecotype (B = beach, E = estuary, Due south = spring) and geography (T = Troms, 50 = Lofoten), see Table 1. The dotted line indicates a geographical split between the ii sampling areas, Troms and Lofoten.
From a TREEMIX analysis, based on 5,982 SNPs and including an outgroup population from Scotland (Aberdeenshire), a tree with no migration events was chosen (Fig. five; adding individual migration events did not significantly improve the overall residual plot). The tree corresponded well with the STRUCTURE results and further indicated the beach ecotype as the ancestral ecotype, two origins of the estuary ecotype (one in each geographical area), and at least 2 origins of the leap ecotype. Ane of these gave rising to the two spring populations in Troms together with the isolated spring population in Lofoten (SL2); the latter, however, on a long branch confirming its distinctiveness. The second Lofoten population (SL1), which grouped with the basal beach populations, may have a divide recent origin. Alternatively, this population may exist so heavily influenced from gene catamenia with the nearby embankment populations and isolated from other leap populations for such a long time that it appears genetically closer to the embankment ecotype.
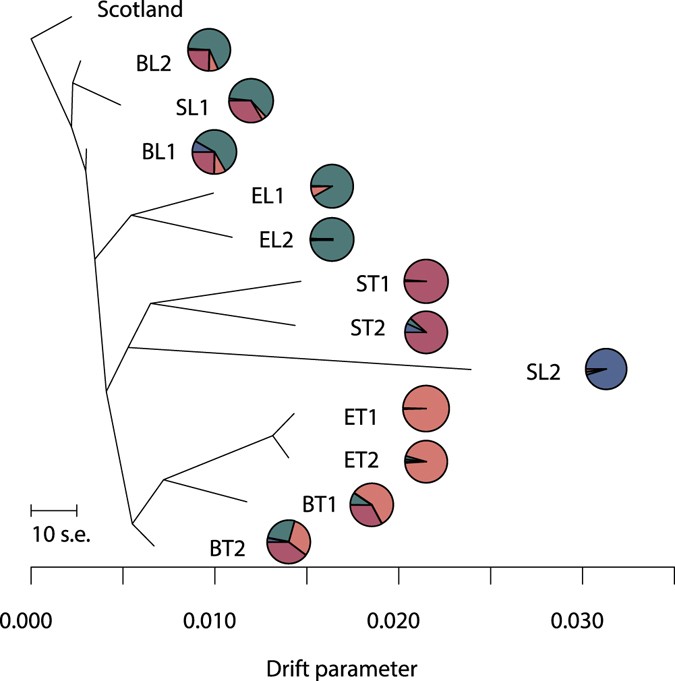
TREEMIX performed on 12 populations of tetraploid Cochlearia officinalis from Northern Norway, using 5,982 SNPs from the RADseq information. A population from Scotland (Aberdeenshire) was used every bit outgroup. Overall assignment of populations to the four Structure groups is visualized by pie charts (corresponding to Fig. 2a). Populations are named according to ecotype (B = beach, Eastward = estuary, S = spring) and geography (T = Troms, L = Lofoten), see Tabular array 1.
A BAYESCAN analysis testing for outlier (RADseq) loci potentially differentiating the beach from the estuary ecotype identified 36 loci within Lofoten and 38 inside Troms, with three of the loci in common (Supporting Data Table S5, Fig. S4a). The analysis testing for outlier loci potentially differentiating the beach from the jump ecotype identified 31 loci within Lofoten and 32 inside Troms, with four common outliers (Supporting Information Tabular array S5, Fig. S5a). Annotations of the mutual outliers (Table 2) indicated genes of potential physiological and ecological relevance, e.g. NHX1 and GABA-T that regulate common salt and drought stress tolerance. GO enrichment tests for biological processes and molecular functions was obtained for each comparing, simply trivial to no overlap was plant between pairwise comparisons in different geographical areas (Supporting Data Figs S4b,c and S5b,c).
Microsatellite analyses
Half dozen microsatellites developed for other Brassicaceae taxa (Arabidopsis, Brassica and Draba; Supporting Information Table S6) were successfully co-amplified in C. officinalis and were further used to analyse the 12 populations from Northern Norway. The six microsatellites each had from 2 to 39 alleles, and in full 98 alleles were scored for 120 individuals. Even though the FIS values calculated from the microsatellites varied more between populations (Supporting Information Table S2), they were also slightly negative or close to cypher (in populations ST1 and EL2 FIS estimated with microsatellites were even lower than RADseq-derived estimates), indicating slight excess of heterozygotes. A STRUCTURE analysis resulted in similar, though less distinct, patterns of genetic variation equally obtained from RADseq data. The degree of admixture varied considerably betwixt single individuals inside a population. Based on deltaK, K = 3, followed by K = seven were suggested as representative number of groups (Supporting Information Fig. S6). When K = 4 was selected (for comparison with the four RADseq groups), and population affiliation to STRUCTURE groups was plotted on the map of Northern Kingdom of norway (Fig. 2c), a like geographical pattern as for the RADseq data was seen, despite differences in the caste of admixture in unmarried populations, and overall more admixture between Troms and Lofoten (Fig. second). The primary difference compared to K = three was that the isolated bound population from Lofoten (SL2) came out as a distinct group.
Discussion
Overall, the genetic patterns amid Cochlearia populations in Northern Norway support differentiation among ecotypes as previously suggested based on morphological and ecological investigations16. Preferences for different types of moist habitats are seen throughout the genus and local accommodation to divergent ecologies has probably been an of import commuter for speciation12.
The analyses suggest the beach ecotype as the ancestral C. officinalis ecotype in Northern Norway. This is supported past its intermediate position in the ordination plot, the relatively short branches in the network, and the high number of private markers. Also, the historical relationships, equally displayed by the TREEMIX analysis, fit a scenario where the bequeathed embankment ecotype from the Lofoten area dispersed to Troms and in parallel locally adjusted to the estuary and spring habitats. The Lofoten/Vesterålen area is one of the areas where the ice withdrew fairly early on from Northern Scandinavia29, xxx, supporting an early on colonisation of C. officinalis from the south/southwest to this area.
Ecotypic differentiation in coastal versus inland habitats is establish in many plant speciesiv, 5. In the genus Grindelia, coastal, inland and intermediate ecotypes prove similar levels of genetic differentiation to what we find in C. officinalis in Northern Norway31. Fragmented or patchy populations will potentially suffer from reduced gene menses betwixt populations and increased genetic differentiation32. Whereas the exposed beach habitat of C. officinalis can be considered more or less continuous following the coastline, the estuary and spring habitats are typically more patchy and isolated. Spring vegetation types are thus described as "small islands in the mural"33. The fragmentary nature of estuary and spring habitats, in combination with limited dispersal, can explain the patterns of strong population amalgamation observed within each of the 2 sampling areas. Several species growing in littoral habitats have seeds that bladder well and are adjusted to dispersal by sea currents over long distances34. In other coastal species, the seeds float less well and dispersal is dependent on the speed of the ocean current35, 36. Dispersal of Cochlearia seeds has non been studied extensively, but they have no apparent adaptation for long altitude dispersal and floating experiments indicate that dispersal with bounding main currents is limited to shorter distances37, 38. With putative limited ocean dispersal, it is not surprising that we find potent population affiliation of the C. officinalis populations and only limited factor exchange, primarily betwixt geographically close populations.
The estuary habitat is connected to the sea, but notwithstanding clearly separated from the more than exposed habitat of the beach ecotype further out in the fjords. The streams might, however, lead to a more or less mutual seed pool. The Cochlearia plants in Northern Norway are obligate outcrossers11, and crossings between ecotypes resulted in seeds with high formation ratexvi. The distinctiveness of the beach and estuary ecotypes is probably related to selection to the rather special estuary habitat, which is characterised by brackish water conditions, regular inundation, and low levels of food and organic textile. Alternatively, differences in flowering time and temporal isolation between ecotypes could be a meaning barrier to gene mensesfive, 39. In controlled experiments, plants of the estuary ecotype showed a trend to delayed flowering compared to the beach ecotype16, but generally plants of both ecotypes take a prolonged flowering period throughout the whole summer and temporal isolation is less likely to explicate the distinctiveness between plants in these two habitats.
Even though the estuary ecotype is morphologically the about distinct of the iii ecotypes16, the jump ecotype turned out to be genetically the nigh singled-out. Genetical distinctness is, however, related to degree of geographical isolation (Fig. S3), with the small isolated forest spring populations (Sørfjorddalen in Lofoten and Kvaløysletta in Troms) genetically most distinct, suggesting that drift may exist an important strength shaping the genetic patterns in this system. But also in this case, it is reasonable to assume that choice to the special spring habitat plays an important role for the distinct characteristics of the plants growing there. Compared to the exposed beaches and the ofttimes inundated estuary habitat, where an almanac or biennial life history is virtually optimal, the leap habitat is much more than sheltered and supportive of perenniality as usually found in the leap ecotype16.
Local accommodation occurs when a population evolves traits that back up higher fitness in its native environment relative to populations from foreign environments40. Genetic differences betwixt populations from contrasting environments tin can be indicative of selection for local adaptation, especially if these patterns are replicated. However, celebrated demographic events tin generate like patterns and ideally one should take fettle information from reciprocal transplant experiments to examine the genetics of local adaptation. In the absence of such information, combination of genetic differences and information about quantitative trait variation tin can be used equally indirect evidence for the role of selection and may help to identify patterns of local adaptation41. Differentiation between forms can have occurred multiple times in situ (parallel development) or as a result of a single origin with subsequent dispersal to areas with suitable habitat7. In addition to the Grindelia example already mentioned31, other recent examples of parallel ecotypic differentiation have been shown in Eucalyptus globules 39 and Senecio lautus 7, with coastal ecotypes originating polytopically from more widespread, inland ecotypes. Our data, combined with previous morphological, ecological and eco-physiological studies of C. officinalis, build a good example of parallel ecotypic divergence as a result of repeated adaptation to the estuary and spring habitats. Analyses of both the SNP and the microsatellite data testify differentiation amongst ecotypes, but too geographical separation within ecotypes, peculiarly for the estuary and bound ecotypes which by the TREEMIX analysis are suggested to have originated polytopically from the ancestral embankment ecotype. Cases of parallel evolution occurring within a species are important for understanding the interaction of natural selection, gene flow and geography on the origin of ecotypes. In our example, these factors or processes have most probable interplayed to produce the genetic patterns that we find among populations of the 3 ecotypes.
1 of the intriguing challenges in ecological genomics is to place the genes that underlie local adaptation. Cases of parallel ecotypic differentiation may provide particular good opportunities to search for candidate genes responding to natural selection, and allow for disentangling the effects of selection and migrate. 1 common way to screen for adaptive loci is FST-based outlier tests, which assume neutral genetic migrate to touch on the entire genome, whereas adaptive loci would be expected to show excess differentiation (outliers) among populations42, 43. A FST-based outlier exam of pairwise comparisons between C. officinalis ecotypes (beach vs. estuary and beach vs. leap) for each sampling area resulted in several candidate loci. Many of these are most likely the effect of migrate merely for some outlier loci, nosotros found a match betwixt comparisons in Troms and Lofoten equally would exist expected if these are adaptive loci, or linked to adaptive loci that have evolved in parallel in the two areas. A couple of outlier loci establish in both beach-estuary comparisons (Tabular array 2), correspond indeed to genes (NHX1, GABA-T) that are known to be involved in table salt tolerance in Arabidopsis and other plants, and could exist important for adaptation to stagnant conditions44,45,46,47. In most cases, the traits that confer local adaptations are polygenic quantitative traits41, and identification of loci that govern variation in such traits is a challenging task and will require a genomic region-based approach that tin observe genetic hitchhiking regions48. Further, the common isolating traits acquired in different populations as a result of parallel ecotypic differentiation may not necessarily exist governed by the same mutation, gene or even pathway in different replicates49. In any case, identification of genes that play a role in adaptation will require selection experiments in controlled and field environments to directly measure their furnishings on fitness, and in addition functional gene analyses to discover loci that actually alter fitnessseven, 48.
Equally RADseq is simply a representation of the genome, important regions of the genome, and thus also several loci potentially involved in adaptive divergence, are about probable disregardedl, 51. Lowry et al.27 estimated the median density of markers from recent studies performing genome scans with RADseq to be 4.08 RADtag per megabase. With haplotypes being one to three orders of magnitude shorter for many species, they concluded that RADseq will miss many loci nether pick. With a density of marker interpretation of 7.26 RAD loci/Mbp for the current written report, we have probably merely been able to identify a minor portion of the bodily number of markers that could be under pick as part of the diversification between the ecotypes of C. officinalis. However fifty-fifty with the limitations of the current approach (RADseq), nosotros were able to identify ecologically-relevant loci that could be involved in divergent adaptation.
Determining the genes and the genetic architecture of traits involved in adaptive divergence between ecotypes is crucial to understand the process of speciation. A common intermediate stage in the process towards reproductive isolation is the development of partially reproductively isolated ecotypes, resulting from adaptation to different habitats. Despite existence isolated ecologically, or partly so, the Cochlearia ecotypes still represent a quite early on stage in this process where reproductive isolation has non nonetheless evolved. Whether or not the ecotypes may eventually become fully distinct species, studying them may give us the opportunity to find the processes leading to diversification. In already diverged and well diagnosed species, these processes are usually even more than obscured. Young incipient species are more likely to display signatures of selective sweeps that can point to disproportion in pick between habitats52. Sequenced annotated genomes would open upwardly for this and also for detailed investigations regarding the possible link between autopolyploidy and rapid phenotypic diversification. In this calorie-free, the autotetraploid Cochlearia in Northern Norway represents an interesting example of parallel ecotypic divergence, illustrated by non-random organisation of genetic variation across the mural that may, or may not, in fourth dimension become reproductively isolated species.
Materials and Methods
Institute Material and Dna extraction
Two populations of each of the three Cochlearia ecotypes were collected from each of the two areas in Northern Norway: Troms and Lofoten (Table 1, Fig. 2). From each population, leaf tissue of 10 individuals was stale and stored in silica gel. When available, mature seeds were also sampled, preferably from the aforementioned individuals. 5 representative individuals from each population were collected as herbarium vouchers and deposited at the herbarium of the Natural History Museum, University of Oslo (O). To obtain fresh leaf tissue for catamenia cytometry, seeds were germinated and plants grown in controlled growth chambers at the University of Oslo (18 h light at 18 °C; 6 h night at x °C). To confirm that the sampled plants were tetraploids, representative plants from all populations (altogether 54 individuals, Tabular array 1) were analysed by flow cytometry to obtain relative nuclear DNA amounts (run across Supporting Information Methods S1 for further details on how the flow cytometry analyses were performed).
DNA was extracted from c. 30 mg silica-dried leafage tissue from each individual with the E.Z.Due north.A. SP Found DNA Kit (Omega bio-tek), following the protocol for dry out samples with modest modifications. Before extraction, the samples were crushed for one–2 min at 20 Hz with 2 3 mm tungsten carbide beads in a tissuelyser Retsch MM301 (Qiagen). In virtually cases, elution with 50 µl (run through once or twice) was used. Earlier RADseq, the Dna samples were cleaned with NucleoSpin gDNA Clean-up (Macherey-Nagel).
RADseq analyses
RADseq libraries were prepared by single assimilate reactions using PstI, combinatorial inline barcoding, and size selection with magnetic beads. The protocol was adapted from previous studies53, 54, with modifications equally indicated below. Altogether 120 Cochlearia samples were included in 2 libraries (lx samples in each); of these 91 (89 individuals and two library replicates), representing the 12 populations from Northern Norway, were included in the information analyses for this study. For each individual, 125 ng Dna was digested at 37 °C for 2 h with xv U PstI-HF (Nib). To remove PstI-HF (which cannot be heat inactivated), all samples were cleaned using SPRIselect Reagent Kit (Beckman Coulter) with no size selection. Later ligation of P5 adapters, samples with different P5 barcodes were pooled together in five sublibraries and sheared by sonication using a Bioruptor Pico (Diagenode) with 3 cycles of 45 southward "on" and 60 due south "off" at 4 °C to achieve an average size of c. 400 bp. Samples were purified with MinElute Reaction Cleanup Kit (Qiagen) followed by left (0.7x) and right (0.55x) side size choice with SPRIselect Reagent Kit. Later on ligation of P7 adapters, like cleaning and size pick, this time just on the left side (0.65x), were performed both before and after PCR amplification with Phusion Main Mix (Beak). The libraries were sent to paired-end sequencing, each in ane Illumina HiSeq2000/HiSeq2500 lane (100 bp/125 bp) at the Norwegian Sequencing Centre, Oslo, Norway (http://www.sequencing.uio.no/).
Raw Illumina reads were processed with STACKS v. 1.2355, 56. To demultiplex the individuals and remove low quality data, the program process_radtags was run with the following settings: PstI as restriction enzyme, removal of any read with an uncalled base, discarding reads with low quality scores, and rescuing barcodes and RADtags. After reads from the ii libraries were cut to the same length (94 bp), ustacks, cstacks and sstacks were run with just forward reads. Different values for m (minimum number of identical raw reads required to create a stack), M (number of mismatches immune between loci when processing a single individual) and n (number of mismatches allowed between loci when building the catalog) were tested to find the settings that maximised the number of reliable loci identified from the reads (see Supporting Information Methods S2 for further details). The settings used in the terminate were thou = 3, K = iv and north = ane. To further optimise the pipeline for tetraploids, each private was allowed to have four alleles (plus one extra to account for potential sequencing errors) by setting the–max_locus_stacks to v (default is 3 when expecting diploids). The export_sql.pl script was used to create a whitelist of loci that contained 1–10 SNPs (snps_l = 1 and snps_u = 10). The program populations was used to link the individuals to their respective population and to produce structure-, vcf-, phylip- and haplotype files, each optimised for a specific purpose. Except for the haplotype file, only one SNP per locus was retained (i.e. the showtime SNP on each locus) to minimise as much as possible linkage of markers. STACKS is, at least at this point, unable to write out total polyploid genotypes, hence our final filtered datasets were diploid-similar. A dandy majority of SNPs are, all the same, expected to be bi-allelic at the population level, significant that information technology is merely information nigh partial heterozygotes which is lost. Information near the filters used (per centum of individuals and populations required for a locus to be processed) and the number of SNPs obtained in each case can be found in Supporting Information Table S1. As replicated samples clustered together in the initial information analyses, only one per accession was included in the final analyses. The vcf file was converted to the appropriate format with PGDSpider v. 2.0.8.257 for analyses done in GENEPOP 5. 4.258, 59 and ARLEQUIN v. iii.5.2.2threescore.
The number of individual alleles and the inbreeding coefficient (FIS) for ecotypes and single populations were obtained from running the plan populations in STACKS. GENEPOP was used to calculate the number of migrants based on private alleles (Nm) for pairwise comparisons of single populations (corrected for size). Violin plots summarising Nm values were constructed using the library vioplot (available from https://CRAN.R-project.org/packet=vioplot) in R v. 3.one.ii. AMOVAs were run in ARLEQUIN to estimate genetic differentiation amongst populations and amongst college level groups (ecotypes and geographical areas). Analyses using ecotypes were run separately for the 2 geographical areas. The assay for Lofoten was done both with and without the bound population SL1 (Himmeltind), as this population turned out to be genetically more than like to the beach populations than to the other spring populations.
The construction file was used to perform a PCA with the R bundle adegenet 5. 1.4–161, 62 in R five. iii.ii.563 using allele frequencies centred to mean zero and scaled, missing values treated equally zero, and Euclidean equally distance measure out. One individual (MKB12–4–17) had a slight outlier position and was removed in the terminal PCA to permit for better resolution of the remaining individuals. Population structure was further investigated with STRUCTURE v. ii.three.364 using the admixture model and correlated frequencies. A tetraploid input file was constructed by using the recessive allele option65 and ploidy ready to iv to permit for ambiguity in partial polyploid heterozygotes66. The analysis was run with K = 1–13, ten runs for each M, 1 1000000 iterations and burn-in of 100,000 using the Lifeportal at the University of Oslo (https://lifeportal.uio.no/). Results were summarised in Construction HARVESTER web v. 0.9.9467 and CLUMPAK beta v.68, producing likelihood and deltaK graphs69. The optimal number of groups converged to the same solution for all replicate runs (confirmed by inspecting the plots) and was visualised using DISTRUCT five. i.i70 and as pie charts on a map of Northern Norway using QGIS five. 2.four.071. The map layer was extracted from GADM version ane.072.
The phylip file was used to produce a phylogenetic network in SPLITSTREE473. Splits were created from Jukes Cantor distances and visualised as a neighbour net with each end node representing an individual. TREEMIX 5. 1.1274 was used to address historical relationships between populations. Using VCF-tools v. 0.1.1275 and PLINK v. ane.9076, the vcf file was converted to a frequency file that could exist transformed to a treemix file using the plink2treemix script available for TREEMIX (https://bitbucket.org/nygcresearch/treemix/downloads). TREEMIX was run with a Scottish C. officinalis population from Aberdeenshire (included in the RADseq libraries) as outgroup, visualised in R and illustrated in combination with the Construction pie charts. The number of migration events was tested by starting at zero and calculation one past one until the residual plot stopped improving.
To detect possible RADseq loci under pick, BAYESCAN v. two.ane42, 43, 77 was used with default settings. The haplotype file produced from populations was used together with a python script to create the input file (containing haplotype information) for BAYESCAN78. Ecotypes were tested in pairwise comparisons betwixt the likely ancestral ecotype (beach) and the estuary and jump ecotypes, respectively. Tests were performed for each geographical area (Troms and Lofoten) separately and then compared to wait for possible common outlier loci. The program sort_read_pairs.pl in STACKS was used to collect the opposite reads (the read pairs) of the outlier loci, and of one,000 random loci of the catalog in order to construct a reference set for further enrichment analyses. The program exec_velvet.pl in STACKS was used to extend the contigs of the outlier and reference loci. The outlier loci for each comparison and the reference ready were annotated and used for further Get enrichment analyses in BLAST2GO five.3.2.779. Fisher'southward exact tests were implemented at a threshold p-value of 0.05. The enriched Get terms from each comparison were summarised, applying thinning based on semantic similarity, and visualised with REViGO80.
The STACKS-pipeline as well as TREEMIX and BAYESCAN analyses were run on the Abel cluster, endemic by the University of Oslo and the Norwegian metacentre for High Performance Computing (NOTUR).
Microsatellite analysis
The M13-tailing arroyo from Schuelke81 was used to exam twenty primers developed for other Brassicaceae taxa (Arabidopsis, Brassica and Draba; Supporting Information Tabular array S2) for co-amplification in xv Cochlearia individuals. Six microsatellites successfully amplified and were used to analyse x individuals from each of the 12 Cochlearia populations from Northern Norway, post-obit the protocol by Vik et al.82 except that 10 μl PCR reaction volumes were used. The annealing temperature used for each microsatellite afterwards optimisation is given in Supporting Data Table S6. At least five replicates and one negative command were included per 96-well plate.
Microsatellite genotypes (based on allele sizes) were assessed in GENEMAPPER 5. 3.vii (Life Technologies/Applied Biosystems). The automated scoring was manually edited to brand sure that the scoring was plausible, i.e. tetraploids had not more than four alleles, and replicates had identically scored profiles. The R package POLYSAT v. i.three83 was used to construct a tetraploid input file, allowing ambiguity in partial heterozygotes, which was analysed in Structure with the same settings as for the RADseq information. FIS was calculated with SPAGeDI (Spatial Pattern Assay of Genetic Variety) that offers a way to approximate the allele frequencies in polyploids past assuming that each of the alleles in a partial heterozygote has an equal likelihood of existence present more than than once66.
References
-
Nosil, P., Harmon, L. J. & Seehausen, O. Ecological explanations for (incomplete) speciation. Trends Ecol. Evol. 24, 145–156 (2009).
-
Lowry, D. B. Ecotypes and the controversy over stages in the formation of new species. Biol. J. Linn. Soc. 106, 241–257 (2012).
-
Seehausen, O. et al. Genomics and the origin of species. Nat. Rev. Genet. xv, 176–192 (2014).
-
Turesson, G. The genotypical response of the plant species to the habitat. Hereditas 3, 211–350 (1922).
-
Lowry, D. B., Rockwood, R. C. & Willis, J. H. Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Development 62, 2196–2214 (2008).
-
Clausen, J. Stages in the evolution of found species (Cornell University Press, 1951).
-
Roda, F. et al. Genomic evidence for the parallel development of coastal forms in the Senecio lautus complex. Mol. Ecol. 22, 2941–2952 (2013).
-
Koch, M. Mid-Miocene departure of Ionopsidium and Cochlearia and its impact on the systematics and biogeography of the tribe Cochlearieae (Brassicaceae). Taxon 61, 76–92 (2012).
-
Koch, M., Huthmann, M. & Hurka, H. Isozymes, speciation and evolution in the polyploid circuitous Cochlearia L. (Brassicaceae). Bot. Acta 111, 411–425 (1998).
-
Koch, 1000., Hurka, H. & Mummenhoff, K. Chloroplast DNA brake site variation and RAPD-analyses in Cochlearia (Brassicaceae): Biosystematics and speciation. Nord. J. Bot. xvi, 585–603 (1996).
-
Nordal, I. & Laane, One thousand. M. Cytology and reproduction in chill. Cochlearia. Sommerfeltia 11, 147–158 (1990).
-
Koch, Chiliad., Dobeš, C., Bernhardt, K. G. & Kochjarová, J. Cochlearia macrorrhiza (Brassicaceae): A bridging species between Cochlearia taxa from the Eastern Alps and the Carpathians? Institute Syst. Evol. 242, 137–147 (2003).
-
Lid, J. & Lid, D. T. Norsk flora. 7. utgåve ved R. Elven (Det Norske Samlaget, 2005).
-
Gill, E. Conservation genetics of the species complex Cochlearia officinalis 50. south.l. In Britain. Phd thesis (The Academy of Edinburgh, 2007).
-
Nordal, I. & Laane, M. 1000. Taxonomic delimitation inside Cochlearia officinalis southward. lat. with particular give-and-take on the rank of C. anglica (Brassicaceae). Acta Univ. Upsal. Symb. Bot. Upsal. 31, 47–57 (1996).
-
Nordal, I. & Stabbetorp, O. E. Morphology and taxonomy of the genus Cochlearia (Brassicaceae) in Northern Scandinavia. Nord. J. Bot. 10, 249–263 (1990).
-
Gill, J. J. B. Cytogenetic studies in Cochlearia Fifty. (Cruciferae). The origins of C. officinalis 50. and C. micacea Marshall. Genetica 44, 217–234 (1973).
-
Gill, J. J. B. Cytogenetic studies in Cochlearia L. The chromosomal homogeneity inside both the 2n = 12 diploids and the 2n = 14 diploids and the cytogenetic relationship between the two chromosome levels. Ann. Bot. 35, 947–956 (1971).
-
Welch, D. & Welch, M. J. Colonisation by Cochlearia officinalis L. (Brassicaceae) and other halophytes on the Aberdeen-Montrose main road in North-Due east Scotland. Watsonia 22, 190–194 (1998).
-
Nordal, I., Eriksen, A. B., Laane, M. M. & Solberg, Y. Biogeographic and biosystematic studies in the genus Cochlearia in Northern Scandinavia. Acta Univ. Upsal. Symb. Bot. Upsal. 27, 83–93 (1986).
-
Eriksen, A. B. & Nordal, I. Ecotypic differentiation in relation to soil nitrogen in Northern Scandinavian Cochlearia officinalis. Ecography 12, 31–38 (1989).
-
Cires, Due east., Samain, M.-S., Goetghebeur, P. & Prieto, J. A. F. Genetic structure in peripheral Western European populations of the endangered species Cochlearia pyrenaica (Brassicaceae). Plant Syst. Evol. 297, 75–85 (2011).
-
Rucińska, A. & Puchalski, J. Comparative molecular studies on the genetic diversity of an ex situ garden drove and its source population of the critically endangered polish endemic plant Cochlearia polonica E. Fröhlich. Biodivers. Conserv. 20, 401–413 (2011).
-
Cieslak, Due east., Ronikier, Chiliad. & Koch, Yard. A. Western Ukrainian Cochlearia (Brassicaceae) the identity of an isolated edge population. Taxon 56, 112–118 (2007).
-
Koch, M. Genetic differentiation and speciation in prealpine Cochlearia: Allohexaploid Cochlearia bavarica Vogt (Brassicaceae) compared to its diploid ancestor Cochlearia pyrenaica DC. in Frg and Austria. Plant Syst. Evol. 232, 35–49 (2002).
-
Lysak, M. A., Koch, M. A., Beaulieu, J. M., Meister, A. & Leitch, I. J. The dynamic ups and downs of genome size evolution in Brassicaceae. Mol. Biol. Evol. 26, 85–98 (2009).
-
Lowry, D. B. et al. Breaking RAD: an evaluation of the utility of brake site‐associated Dna sequencing for genome scans of adaptation. Mol. Ecol. Resour. 17, 142–152 (2017).
-
Soltis, P. S. & Soltis, D. E. The role of genetic and genomic attributes in the success of polyploids. P. Natl. Acad. Sci. USA 97, 7051–7057 (2000).
-
Rasmussen, A. Late Weichselian moraine chronology of the Vesterålen islands, North Kingdom of norway. Norw. J. Geol. 64, 193–219 (1984).
-
Vorren, T. O. et al. Palaeoenvironment in northern Kingdom of norway between 22.2 and fourteen.5 cal. ka BP. Boreas 42, 876–895 (2013).
-
Moore, A. J., Moore, West. L. & Baldwin, B. G. Genetic and ecotypic differentiation in a Californian found polyploid complex (Grindelia, Asteraceae). PloS 1 nine, e95656 (2014).
-
Young, A., Boyle, T. & Brown, T. The population genetic consequences of habitat fragmentation for plants. Trends Ecol. Evol. eleven, 413–418 (1996).
-
Fremstad, East. & Moen, A. Truete vegetasjonstyper i Norge. NTNU Vitensk.mus. Rapp. Bot. Ser. 2001–iv, i–231 (2001).
-
Skarpaas, O. & Stabbetorp, O. E. Diaspore ecology of Mertensia maritima: furnishings of concrete treatments and their relative timing on dispersal and germination. Oikos 95, 374–382 (2001).
-
Curle, C. M., Stabbetorp, O. E. & Nordal, I. Eryngium maritimum, biology of a plant at its northernmost localities. Nord. J. Bot. 24, 617–628 (2004).
-
Solås, H. F., Stabbetorp, O. Eastward. & Nordal, I. The viability of a establish "on the border": Glaucium flavum (Papaveraceae) in Norway. Nord. J. Bot. 24, 433–444 (2004).
-
Praeger, R. L. On the buoyancy of the seeds of some Britannic plants. Sci. P. Roy. Dublin Soc. 14, thirteen–62 (1913).
-
Quinn, R. Grand., Lawton, J. H., Eversham, B. C. & Woods, S. N. The biogeography of deficient vascular plants in Britain with respect to habitat preference, dispersal power and reproductive biology. Biol. Conserv. 70, 149–157 (1994).
-
Foster, S. A., McKinnon, G. E., Steane, D. A., Potts, B. M. & Vaillancourt, R. E. Parallel development of dwarf ecotypes in the wood tree Eucalyptus globulus. New Phytol. 175, 370–380 (2007).
-
Richardson, J. Fifty., Urban, G. C., Bolnick, D. I. & Skelly, D. Thou. Microgeographic adaptation and the spatial scale of evolution. Trends Ecol. Evol. 29, 165–176 (2014).
-
Savolainen, O., Lascoux, 1000. & Merilä, J. Ecological genomics of local adaptation. Nat. Rev. Genet. 14, 807–820 (2013).
-
Foll, M. & Gaggiotti, O. A genome-browse method to place selected loci appropriate for both dominant and codominant markers: a Bayesian perspective. Genetics 180, 977–993 (2008).
-
Foll, Chiliad., Fischer, Grand. C., Heckel, G. & Excoffier, 50. Estimating population structure from AFLP distension intensity. Mol. Ecol. xix, 4638–4647 (2010).
-
He, C. et al. Expression of an Arabidopsis vacuolar sodium/proton antiporter cistron in cotton improves photosynthetic performance nether salt weather condition and increases fiber yield in the field. Plant Cell Physiol. 46, 1848–1854 (2005).
-
Renault, H. et al. ϒ-Aminobutyric acid transaminase deficiency impairs central carbon metabolism and leads to cell wall defects during salt stress in Arabidopsis roots. Plant Cell Environ. 36, 1009–1018 (2013).
-
Adabnejad, H., Kavousi, H. R., Hamidi, H. & Tavassolian, I. Assessment of the vacuolar Na+/H+ antiporter (NHX1) transcriptional changes in Leptochloa fusca L. in response to salt and cadmium stresses. MBRC 4, 133–142 (2015).
-
Pehlivan, Northward. et al. Co-overexpressing a plasma membrane sodium/proton antiporter and a vacuolar membrane sodium/proton antiporter significantly improves salt tolerance in transgenic Arabidopsis plants. Found Cell Physiol. 57, 1069–1084 (2016).
-
Kubota, S. et al. A genome browse for genes underlying microgeographic-scale local adaptation in a wild Arabidopsis species. PLoS Genet. eleven, e1005361 (2015).
-
Ostevik, Thou. L., Moyers, B. T., Owens, K. L. & Rieseberg, L. H. Parallel ecological speciation in plants? Int. J. Ecol. 2012, 939862 (2012).
-
Davey, J. W. et al. Special features of RAD Sequencing data: implications for genotyping. Mol. Ecol. 22, 3151–3164 (2013).
-
Tiffin, P. & Ross-Ibarra, J. Advances and limits of using population genetics to empathise local adaptation. Trends Ecol. Evol. 29, 673–680 (2014).
-
Andrew, R. L. & Rieseberg, Fifty. H. Divergence is focused on few genomic regions early in speciation: incipient speciation of sunflower ecotypes. Evolution 67, 2468–2482 (2013).
-
Baird, North. A. et al. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS I three, e3376 (2008).
-
Paun, O. et al. Processes driving the adaptive radiations of a tropical tree (Diospyros, Ebenaceae) in New Caledonia, a biodiversity hotspot. Syst.Biol. 65, 212–227 (2016).
-
Catchen, J. K., Hohenlohe, P. A., Bassham, South., Amores, A. & Cresko, West. A. Stacks: an assay tool set for population genomics. Mol. Ecol. 22, 3124–3140 (2013).
-
Catchen, J. Thou., Amores, A., Hohenlohe, P., Cresko, Westward. & Postlethwait, J. H. Stacks: building and genotyping loci de novo from short-read sequences. G3 Genes Genomes Genet. 1, 171–182 (2011).
-
Lischer, H. E. 50. & Excoffier, L. PGDSpider: an automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics. 28, 298–299 (2012).
-
Raymond, Yard. & Rousset, F. GENEPOP (version 1.2): population genetics software for verbal tests and ecumenicism. J. Hered. 86, 248–249 (1995).
-
Rousset, F. genepop'007: a complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 8, 103–106 (2008).
-
Excoffier, L. & Lischer, H. E. L. Arlequin suite ver iii.v: a new serial of programs to perform population genetics analyses nether Linux and Windows. Mol. Ecol. Resour. 10, 564–567 (2010).
-
Jombart, T. adegenet: a R bundle for the multivariate analysis of genetic markers. Bioinformatics 24, 1403–1405 (2008).
-
Jombart, T. & Ahmed, I. adegenet 1.iii-one: new tools for the analysis of genome-broad SNP data. Bioinformatics 27, 3070–3071 (2011).
-
R Cadre Team. R: A linguistic communication and environment for statistical computing. R Foundation for Statistical Computing http://www.R-projection.org/(2016).
-
Pritchard, J. One thousand., Stephens, K. & Donnelly, P. Inference of population construction using multilocus genotype data. Genetics 155, 945–959 (2000).
-
Falush, D., Stephens, M. & Pritchard, J. 1000. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol. Ecol. Notes seven, 574–578 (2007).
-
Dufresne, F., Stift, One thousand., Vergilino, R. & Mable, B. One thousand. Recent progress and challenges in population genetics of polyploid organisms: an overview of current country-of-the-art molecular and statistical tools. Mol. Ecol. 23, xl–69 (2014).
-
Earl, D. A. & vonHoldt, B. M. Structure Harvester: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361 (2012).
-
Kopelman, Due north. M., Mayzel, J., Jakobsson, 1000., Rosenberg, Due north. A. & Mayrose, I. Clumpak: a program for identifying clustering modes and packaging population construction inferences across Thou. Mol. Ecol. Resour. 15, 1179–1191 (2015).
-
Evanno, Thousand., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14, 2611–2620 (2005).
-
Rosenberg, North. A. Distruct: a program for the graphical brandish of population construction. Mol. Ecol. Notes 4, 137–138 (2004).
-
QGIS. QGIS Geographic Information System. Open up Source Geospatial Foundation Project http://world wide web.qgis.org/ (2015).
-
GADM. GADM database of Global Administrative Areas, version 1.0. http://www.gadm.org/ (2009).
-
Huson, D. H. & Bryant, D. Awarding of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267 (2006).
-
Pickrell, J. K. & Pritchard, J. 1000. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet. viii, e1002967 (2012).
-
Danecek, P. et al. The variant telephone call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
-
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. AJHG 81, 559–575 (2007).
-
Fischer, M. C., Foll, M., Excoffier, L. & Heckel, K. Enhanced AFLP genome scans notice local adaptation in loftier-altitude populations of a minor rodent (Microtus arvalis). Mol. Ecol. 20, 1450–1462 (2011).
-
Trucchi, E., Frajman, B., Haverkamp, T., Schönswetter, P. & Paun, O. Genomic and metagenomic analyses reveal parallel ecological departure in Heliosperma pusillum (Caryophyllaceae). bioRxiv, doi:10.1101/044354 (2016).
-
Conesa, A. et al. Blast2GO: a universal tool for annotation, visualization and assay in functional genomics inquiry. Bioinformatics 21, 3674–3676 (2005).
-
Supek, F., Bošnjak, Chiliad., Škunca, North. & Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PloS one 6, e21800 (2011).
-
Schuelke, M. An economical method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 18, 233–234 (2000).
-
Vik, U., Jørgensen, M. H., Kauserud, H., Nordal, I. & Brysting, A. K. Microsatellite markers show decreasing multifariousness simply unchanged level of clonality in Dryas octopetala (Rosaceae) with increasing breadth. Am. J. Bot. 97, 988–997 (2010).
-
Clark, L. V. & Jasieniuk, M. POLYSAT: an R package for polyploid microsatellite analysis. Mol. Ecol. Resour. 11, 562–566 (2011).
-
Shelp, B. J., Bown, A. W. & McLean, M. D. Metabolism and functions of gamma-aminobutyric acrid. Trends Plant Sci. iv, 446–452 (1999).
-
Hadley, B. et al. Structure and function of nucleotide sugar transporters: electric current progress. CSBJ x, 23–32 (2014).
-
Singh, A. et al. Genome-wide expressional and functional analysis of calcium transport elements during abiotic stress and development in rice. FEBS Journal 281, 894–915 (2014).
-
Wu, K., Gu, Y., Li, Due south. & Yang, Z. A genome-broad analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell 13, 2841–2856 (2001).
-
Caffall, Thou. H., Pattathil, S., Phillips, S. E., Hahn, M. Chiliad. & Mohnen, D. Arabidopsis thaliana T-DNA mutants implicate GAUT genes in the biosynthesis of pectin and xylan in cell walls and seed testa. Mol. Plant two, 1000–1014 (2009).
-
Matsushita, A., Furumoto, T., Ishida, South. & Takahashi, Y. AGF1, an AT-hook protein, is necessary for the negative feedback of AtGA3ox1 encoding GA three-oxidase. Found Physiol. 143, 1152–1162 (2007).
Acknowledgements
Nosotros give thanks Pavel Trávníček for running the flow cytometry analyses, Anna Mazzarella and Emiliano Trucchi for help with initial STACKS analyses and python scripts, and Charlotte Bjorå and Odd Stabbetorp for discussions on Cochlearia. The projection was supported by the Nansen Foundation, Systematics Research Fund, Southward.Chiliad.Sønneland Foundation, and Professor Rathke, Professor Collett and Professor Wille'south Legacy. Thou.Thousand.B., Grand.T.L and O.P were in part funded through an Austrian Scientific discipline Fund project (FWF Y661-B16).
Author data
Affiliations
Contributions
Thou.Chiliad.B., A.1000.B., O.P. and I.N. planned and designed the inquiry; M.One thousand.B., A.K.B. and I.N. collected the plants; M.Thousand.B. and M.T.50. compiled and processed the data; Chiliad.K.B. analysed the information. M.Yard.B., A.1000.B., O.P. and I.Northward. interpreted the results and wrote the manuscript.
Respective author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher'due south note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary textile
Rights and permissions
Open Access This commodity is licensed under a Creative Eatables Attribution iv.0 International License, which permits use, sharing, adaptation, distribution and reproduction in whatsoever medium or format, as long as you give advisable credit to the original writer(s) and the source, provide a link to the Creative Eatables license, and indicate if changes were fabricated. The images or other third party cloth in this article are included in the commodity'south Creative Commons license, unless indicated otherwise in a credit line to the material. If fabric is not included in the article's Creative Eatables license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you lot will need to obtain permission straight from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/four.0/.
Reprints and Permissions
Near this article
Cite this commodity
Brandrud, Chiliad.K., Paun, O., Lorenzo, Grand.T. et al. RADseq provides testify for parallel ecotypic deviation in the autotetraploid Cochlearia officinalis in Northern Norway. Sci Rep 7, 5573 (2017). https://doi.org/10.1038/s41598-017-05794-z
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-017-05794-z
Comments
By submitting a annotate you agree to bide by our Terms and Community Guidelines. If y'all find something abusive or that does not comply with our terms or guidelines please flag it equally inappropriate.
Source: https://www.nature.com/articles/s41598-017-05794-z